Proudly Serving Our Customers
See All ReviewsBeer Carbonation Guide - Getting It Just Right!
A simple technique to help you get perfect carbonation
Simple Introduction to Beer Carbonation
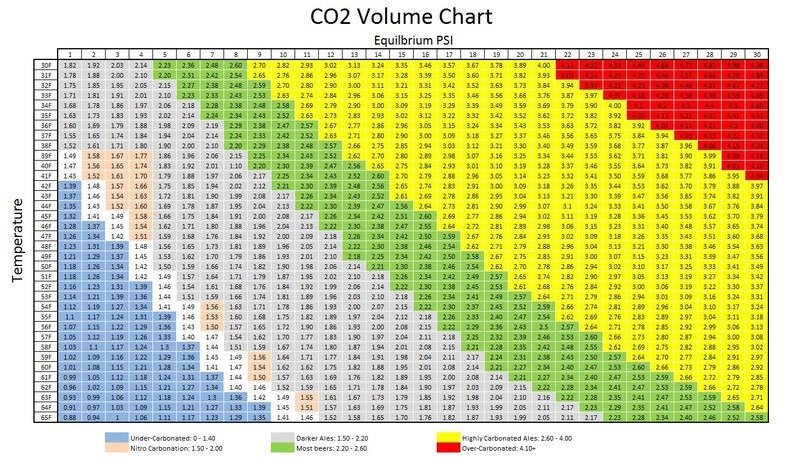
The overall level of carbonation of any beverage product is determined by the volume of dissolved CO2. The CO2 volume can be measured with a variety of hand held as well as scientific instruments. Each device is going to give you a number that corresponds to the chart to the right, which offers optimal ranges of CO2 dissolved volume based various beer styles. The range limits should be used merely as a guide, because at the end of the day nothing is quite as telling as a taste test to determine specific CO2 levels.
Uniform and Rapid Carbonation
The best way to achieve uniform and rapid carbonation is two-fold: first we want to ensure the proper amount of head space which is usually around 10-15%, and secondly, utilizing a carbonation stone rather than alternative methods. A carbonation stone produces tiny micro bubbles that are more readily absorbed by the finished product, particularly when we keep our temperature between 30-33°F and the flow of the CO2 has been optimized. Much like wort aeration, there is a science behind carbonation. We recommend referencing the equilibrium chart to the right which shows the measured PSI of different beers at certain temperatures with their respective volumes of dissolved CO2.
Selecting a Carbonation Stone
Carbonation Stones: The most important part in the carbonation process is the carb stone. At Glacier Tanks we offer a dual use stone (carbonation or oxygen) with a 2 micron porosity. Carbonation stones are most effective from 0.5 to 3 microns. Any smaller and the stone becomes clogged too easily by proteins and organic material, any higher and the bubbles are too large and won't dissolve into the product resulting in uneven foaming of your beer.

Calculating Carbonation Pressure
To calculate our equilibrium PSI, we start with the wetting pressure. The wetting pressure is the PSI we need to produce bubbles on a carbonation stone when wetted. For most stones, this wetting pressure is between 2-8 PSI. To calibrate the stone, submerge it in water at the same orientation as it will be in the tank and slowly increase the PSI until bubbles begin to flow, record the PSI, then slowly decrease the CO2 until the bubbles stop completely. Record this last PSI reading and take the average of these two readings to determine your wetting pressure PSI.
Next, we need to determine the head pressure on the stone itself. The head pressure of the product on the stone will play a role in the pressure needed to carbonate. We start with the assumption that approximately every 28 inches of product will add 1 PSI of pressure against the stone. This PSI needs to be added to the wetting pressure to determine how much total pressure is required to begin producing bubbles inside of the tank. Every tank will be different, so this is merely an approximation and you may need to adjust slightly.
One you have your two pressure values and selected the desired CO2 volume of your final product, we can punch those values in the equation below and get the PSI output needed from your CO2 system:
Wetting Pressure + Head Pressure + desired Equilibrium PSI = Carbonation Pressure
As an example:
- Carbonation Stone Wetting Pressure = 3.0 PSI
- A 20 BBL Brite Tank w/ 18 BBLs (10% head space) that is 68 vertical inches of product, thus 68 in. / 28 in. = 2.43 PSI
- We have a dark Ale with a desired CO2 volume of 2.17 @ 45°F, thus we find an equilibrium PSI = 11
Wetting Pressure (3.0) + Head Pressure (2.43) + Equilibrium PSI (11) = 16.43 PSI
You may round up or down depending on your leanings or aim for a nice middle ground of 14.5.
The Advantage of Simplicity
One of the advantages of using this method of carbonation is that ultimately the physics will kick in and stop your carbonation when you've reached your desired point. The reasoning is that as you continue to add carbonation, this will increase the PSI inside the tank until it reaches an equilibrium: the force against the carbonation stone will be equal to the pressure being pushed into the stone. These forces will cancel out and no further bubbles will form. Your pressure gauge should read your calculated PSI of 14.5 (within a margin of error as gauges fluctuate 1-2 PSI naturally). At this point, your product carbonation is finished.